Traceability on automated lines: Why compliance is not optional?
In the current context of industrial automation, traceability and sensorization have been consolidated as strategic elements to improve efficiency, reduce costs and ensure quality in real time. However, there is one aspect that is often relegated to the background: regulatory compliance.
A few days ago we launched a poll on LinkedIn with the question:
- What do you consider most relevant in the application of traceability and sensing in an automated line? -
The results were revealing:
- Real-time quality control: 42
- Predictive fault diagnosis: 26
- Process optimization: 32
- Regulatory compliance: 0%
The most striking fact is the last one: not a single vote for regulatory compliance. And that gives us pause for thought.
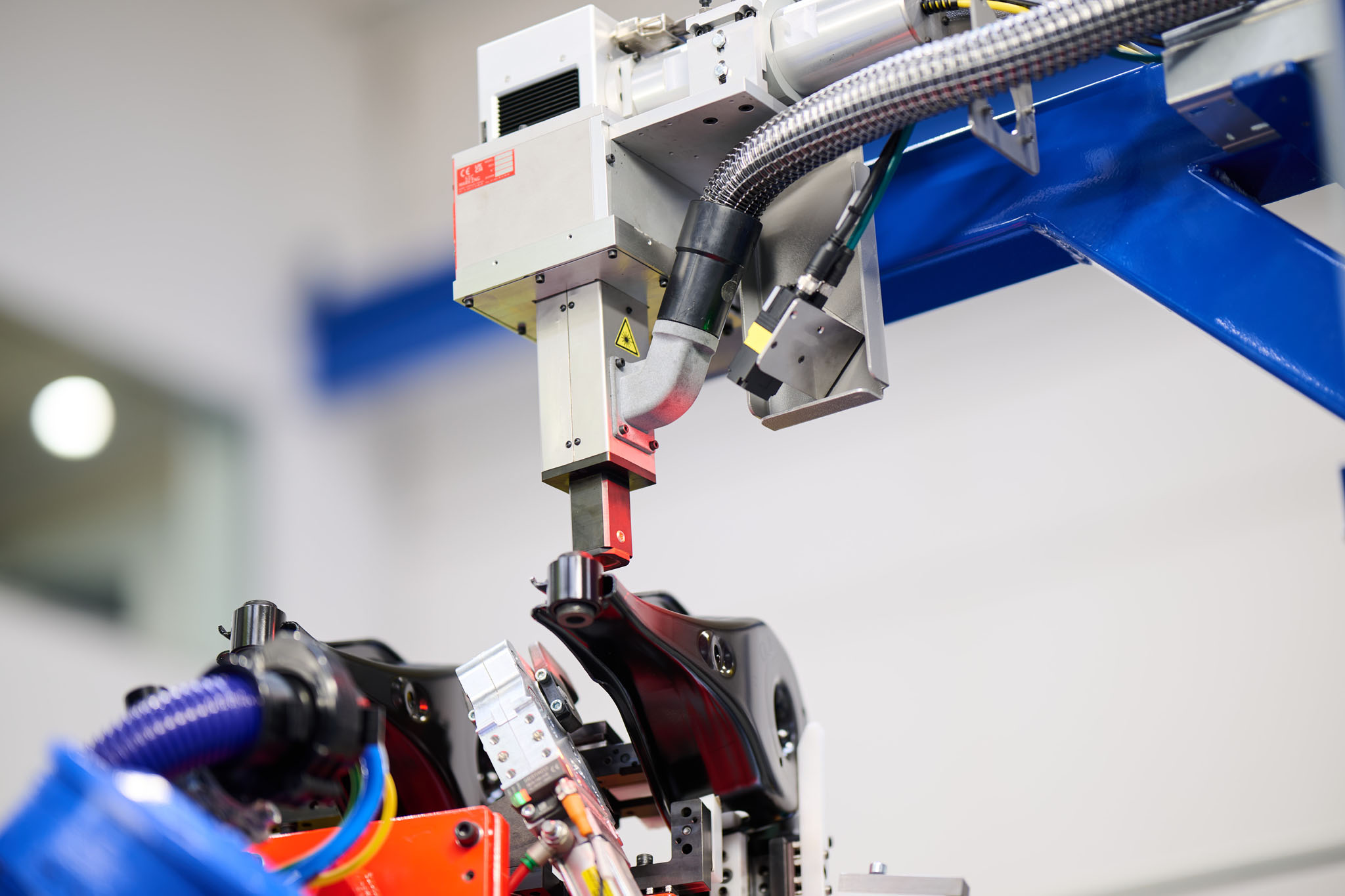
Laser marking in an automated bushing pressing installation.
Why is compliance underestimated?
In many projects, legal and regulatory compliance is perceived as an administrative formality rather than a critical function of automation that adds value or differentiates. It is taken for granted. And yet, it is the basis that legitimizes and protects the entire traceability system, especially in sectors such as food (IFS, BRC, FDA), pharmaceutical (GMP, 21 CFR Part 11), aeronautics (EN 9100) or automotive (IATF 16949).
Without the correct regulatory structuring from the beginning, a line can be technologically advanced but legally unfeasible. And that is a silent risk.
Traceability is not complete if it is not compliant
When designing a sensing or data capture solution in the plant, we must ask ourselves:
- Is the data stored with verifiable digital integrity and traceability?
- Are audit or electronic signature requirements met?
- Is structured retrieval of information possible in case of inspection?
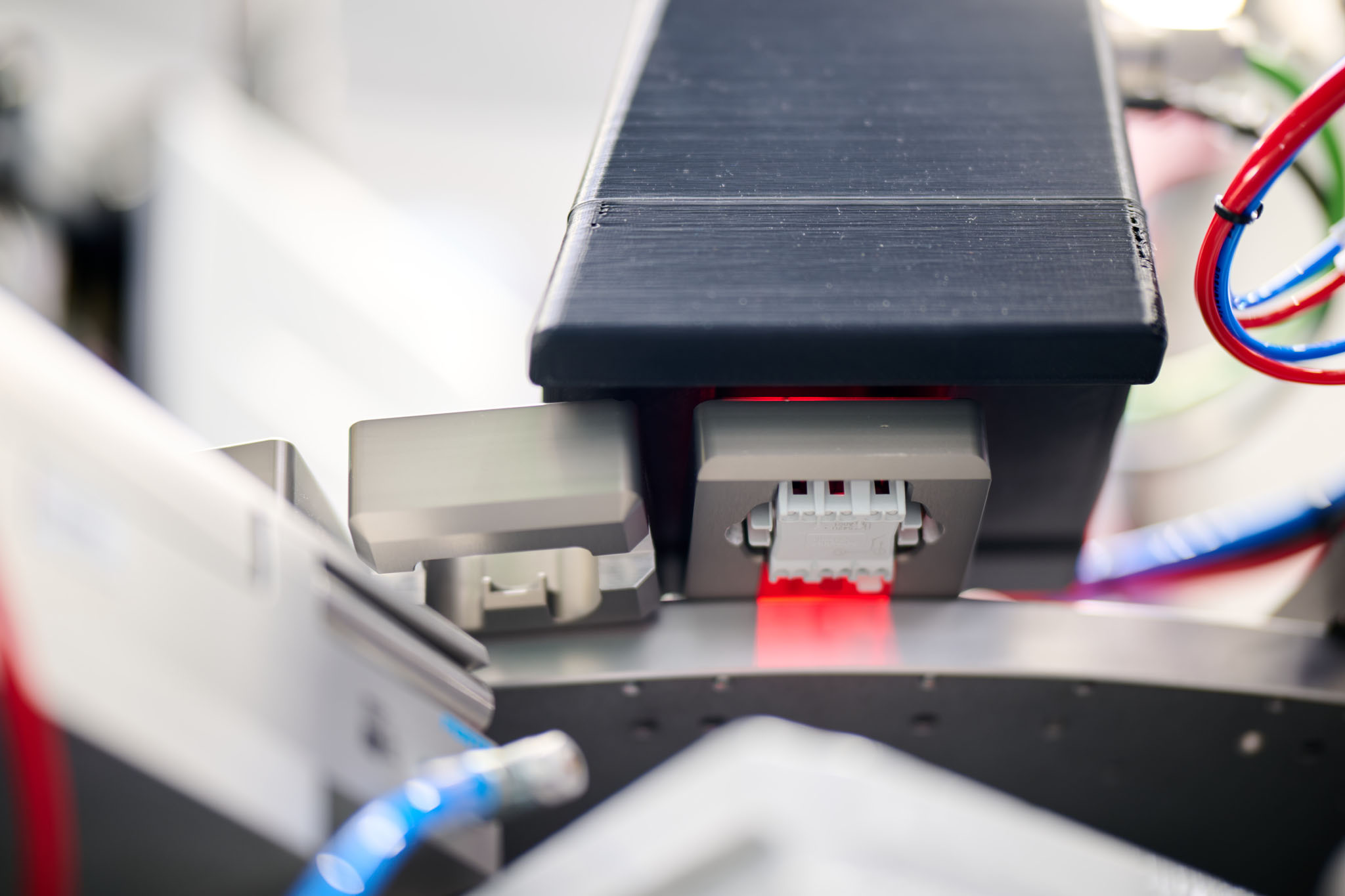
Laser marking in automated installations | Electrical sector.
Poorly designed traceability can fail just when it is needed most: during a quality crisis, a complaint or an external audit. And at that point it's not just about efficiency, but about reputation and business viability.
Regulatory compliance is critical because it ensures that the traceability system supports applicable legal and industry requirements. An automation design without considering these frameworks can result in penalties, product recalls or loss of certifications.
It also helps to standardize processes, facilitate audits and protect both the manufacturer and the end consumer. From the system design stage, functions must be included to ensure reliable, secure and retrievable recording of data required by regulations.
Frequent problems caused by ignoring regulatory compliance
- Delays in audits due to lack of structured records or reliable evidence.
- Unplanned downtime for system revalidation or document redesign.
- Inability to access certain markets where compliant traceability is mandatory.
- Loss of competitiveness against companies with robust certifications.
- Sanctions or product recalls, with their corresponding economic and image impact.
Recommendations for integrating regulations from the design stage
-
Include regulatory requirements in the technical specification of the project, not as an annex, but as a central part.
-
Select sensors and PLCs compatible with electronic logging and validation systems.
-
Implement digital traceability with access control, electronic signatures and encrypted backups.
-
Coordinate from the beginning with those responsible for quality, food safety or compliance.
-
Document the system according to applicable standards, with user manuals, validations and maintenance protocols.

